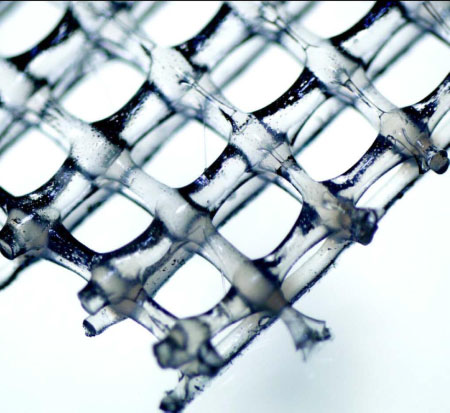
Over the last couple of days, several companies which commercialize 3D printing and/or solution electrospinning machines for the biomaterials, medical device and tissue engineering & regenerative medicine research community but also industry have launched melt electrospinning equipment.
Guidelines to boost productivity
The research community has to analyze the data sheets and the performance track record of those machines carefully if those fulfil the requirements to address the research questions posed in a PhD or larger consortium project. Likewise, the biomedical industry has a critical need for standardized terminology and consistent reporting of parameters related to the machine performance under commercial terms. Thus, this blog will aim to discuss guidelines and herein provide recommendations regarding (1) standardised terminology and units, (2) information to be included in describing the capabilities and performance as well as methods used for the commercial electrospinning equipment with a focus on melt electrospinning.