Hydrogel Chemistry & Crosslinking – Part 5
Michael-Type Crosslinking
Michael type reactions (named after Arthur Michael, 1853-1942) are a broad class of addition reactions in which an unsaturated organic compound (eg vinyl sulfone) reacts with a positively charged (nucleophilic) compound (for example thiols). The reaction conditions can be very mild – ie neutral pH and ambient temperature – perfect for encapsulating living cells.
How does this work?
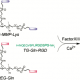
In an example of a typical Michael-type hydrogel, the following two solutions are prepared separately:
- Solution of molecule A: a PEG polymer with vinyl sulfone end groups, and
- Solution of molecule B: a crosslinker molecule, with thiol end groups.
When solution A is mixed with solution B, the vinyl sulfone groups react spontaneously with the thiol groups – resulting in a crosslinked hydrogel network forming. Maleimide is a common alternative to vinyl sulfone. Since the reaction is spontaneous, these hydrogels can be used for injectable systems – the two components are injected separately and crosslink upon mixing.
What about other functionalities?
By changing the properties of the crosslinker molecule (B), other functionalities can be incorporated into the hydrogel. For example, if molecule B is a peptide chain, then it can include certain bioactive peptide sequences, such as cell-adhesion sites, or enzymatically degradable peptide linkages. This flexibility allows different functions, or combinations of functions, to be easily compared in otherwise similar hydrogels.
Are there any disadvantages?
One downside of Michael-type hydrogels is that there is limited control over the reaction kinetics or initiation. Once the two precursor solutions are mixed the reaction begins– so controlling the timing of mixing is critical, especially when the goal is to encapsulate cells or make 3D printed constructs.
Read the previous article in this series. Next up: acrylate-based chemistries.