Hydrogel Chemistry & Crosslinking – Part 4
Enzymatic crosslinking is the way in which our blood clots during wound healing – cells secrete an enzyme, transglutaminase, which crosslinks fibrin, forming an insoluble protein polymer, or clot. By using this enzyme, along with the appropriate functional groups, hydrogels can be crosslinked in much the same way.
Common Factor XIII
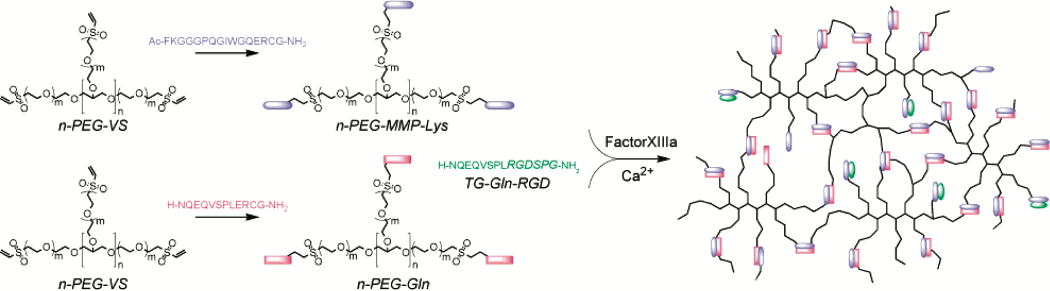
The most commonly used transglutaminase enzyme is Factor XIII, which crosslinks amine groups with acyl groups, which can be found on the amino acids lysine and glutamine, respectively. Therefore, polymers with a mixture of lysine and glutamine can be crosslinked in the presence of Factor XIII. The image below, from (Ehrbar et al. 2007), shows a synthetic, PEG hydrogel system that is crosslinked by Factor XIII. In this example, the hydrogel also has degradation sites, allowing the cells to remodel the hydrogel during regeneration.
Benefits of Enzymatic Crosslinking
A key benefit of enzymatic crosslinking is that the crosslinking occurs under mild, physiological conditions – for example no large temperature changes are required, no toxic chemicals, and no and no (potentially) harmful radiation. The mechanism also doesn’t require light to be able to penetrate through the gel, so opaque gels or constructs can be produced. On the downside, controlling the degree of crosslinking, and the mechanical properties, is more difficult than with other systems, such as photo-crosslinking.
Read the previous article in this series. Next up: Michael-Type Crosslinking
References
Hubbell, Franz E. Weber and Matthias P. Lutolf. 2007. “Biomolecular Hydrogels Formed and Degraded via Site-Specific Enzymatic Reactions.” Biomacromolecules 8: 3000-3007.
Image: Reprinted with permission from Ehrbar et al 2007. Copyright 2007 American Chemical Society.